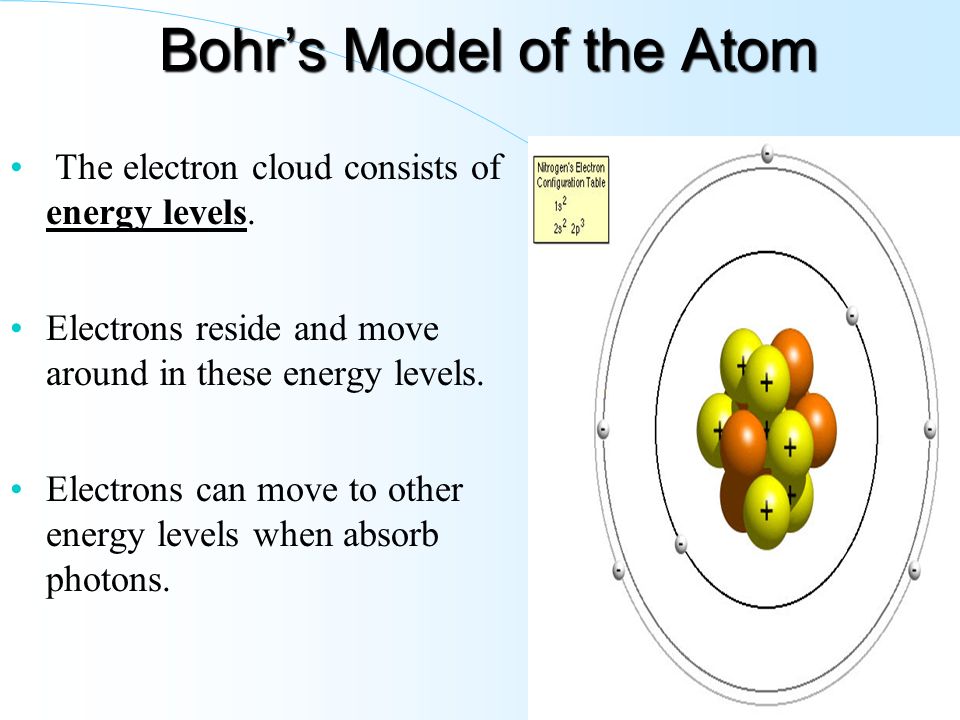
45 what is the term used to label the energy levels of electrons
papers.gceguide.com › O Levels › Chemistry (5070Cambridge O Level - GCE Guide 3 UCLES 2021 5070/21/O/N/21 [Turn over 2 Dry air contains nitrogen, oxygen, noble gases and carbon dioxide. (a) State the percentage of oxygen present in dry air. [1] (b) Carbon dioxide is removed from a sample of air by passing the air through aqueous sodium 8 The Hamiltonian Matrix - The Feynman Lectures on Physics Dear Reader, There are several reasons you might be seeing this page. In order to read the online edition of The Feynman Lectures on Physics, javascript must be supported by your browser and enabled.If you have have visited this website previously it's possible you may have a mixture of incompatible files (.js, .css, and .html) in your browser cache.
Microbial Fuel Cell - an overview | ScienceDirect Topics M. Ruscalleda Beylier, ... R.-C. Wang, in Comprehensive Biotechnology (Third Edition), 2011 6.22.4.1 Microbial Fuel Cells. Microbial fuel cells (MFCs) are a new bioelectrochemical process that aims to produce electricity by using the electrons derived from biochemical reactions catalyzed by bacteria. The energy generated by MFCs is expected to supply enough energy …

What is the term used to label the energy levels of electrons
phet.colorado.edu › en › simulationBuild an Atom - Atoms | Atomic Structure | Isotope ... - PhET Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! › cbse-sample-papers-for-class-10CBSE Sample Papers for Class 10 Science Term 2 Set 2 with ... Feb 10, 2022 · But it would require a large amount of energy to remove four electrons leaving behind a carbon cation with six protons in its nucleus holding on to just two electrons. 2. If carbon accepts 4 electrons, it becomes C 4-. But it would be difficult for the nucleus with six protons to hold on to ten electrons, that is, four extra electrons. structbio.pitt.edu › images › sbl2014Chapter 1 INTRODUCTION TO NMR SPECTROSCOPY or shielded, by the presence of electrons that surround the nucleus, giving a modified field at the nucleus, B: B =(1−σ)B o (1.4) where σ represents the degree of shielding. An extensive discussionofshielding effects is found in Section 1.3. Assuming that the magnetic field is along the z-axis, the energy of each state is: H = −u zB z ...
What is the term used to label the energy levels of electrons. CBSE Sample Papers for Class 10 Science Term 2 Set 2 with … 10.2.2022 · But it would require a large amount of energy to remove four electrons leaving behind a carbon cation with six protons in its nucleus holding on to just two electrons. 2. If carbon accepts 4 electrons, it becomes C 4-. But it would be difficult for the nucleus with six protons to hold on to ten electrons, that is, four extra electrons. › mission-areas › water-resourcesOxidation/Reduction (Redox) | U.S. Geological Survey Feb 27, 2019 · If no dissolved oxygen is present, the water is “anoxic”, but there are other chemical species—nitrate, manganese, iron, sulfate, and carbon dioxide, in that order—that can accept electrons in oxygen’s place. Redox processes typically are enabled by bacteria, which use the energy produced by the processes. › III_088 The Hamiltonian Matrix - The Feynman Lectures on Physics The splitting of the energy levels of the ammonia molecule is, however, strictly a quantum mechanical effect. The splitting of the energy levels of the ammonia molecule has important practical applications which we will describe in the next chapter. Lighting Glossary – Lighting Terms and Definitions | FLOS USA The term is commonly used in theatrical lighting, ... A lighting design strategy that maximizes the use of natural light to reduce energy costs and create indoor spaces that feel natural and appealing. ... Higher illuminance levels make surfaces appear brighter to the human eye and improve visibility.
David Sinclair Recommends GMP NMN – Food Security 22.12.2021 · Inhibition of PARP1 is also used in the treatment of many different kinds of cancers. The fact that administering NMN through the intraperitoneal method increases NAD+ levels – along with the identification of a transporter specific to NMN – means that NMN gets actively transported to the cells to get converted to NAD+. Oxidation/Reduction (Redox) | U.S. Geological Survey 27.2.2019 · If no dissolved oxygen is present, the water is “anoxic”, but there are other chemical species—nitrate, manganese, iron, sulfate, and carbon dioxide, in that order—that can accept electrons in oxygen’s place. Redox processes typically are enabled by bacteria, which use the energy produced by the processes. Unit 6 Flashcards | Quizlet Label the coronal view of the head based on the hints provided. ... Regulation of blood glucose levels. ... Oxidative phosphorylation is a term used to describe... The production of ATP by the electron transport chain. The electrons from NADH are transferred to... Coenzyme Q. EV Battery Recycling | Union of Concerned Scientists 11.2.2021 · Increasing the amount of renewable energy used to charge an electric vehicle, however, results in the most significant reductions in global warming emissions over the life cycle of an EV. A significant portion of demand for battery materials could be met by recycling, transitioning to low-cobalt cathode formulations, and high levels of material recovery.
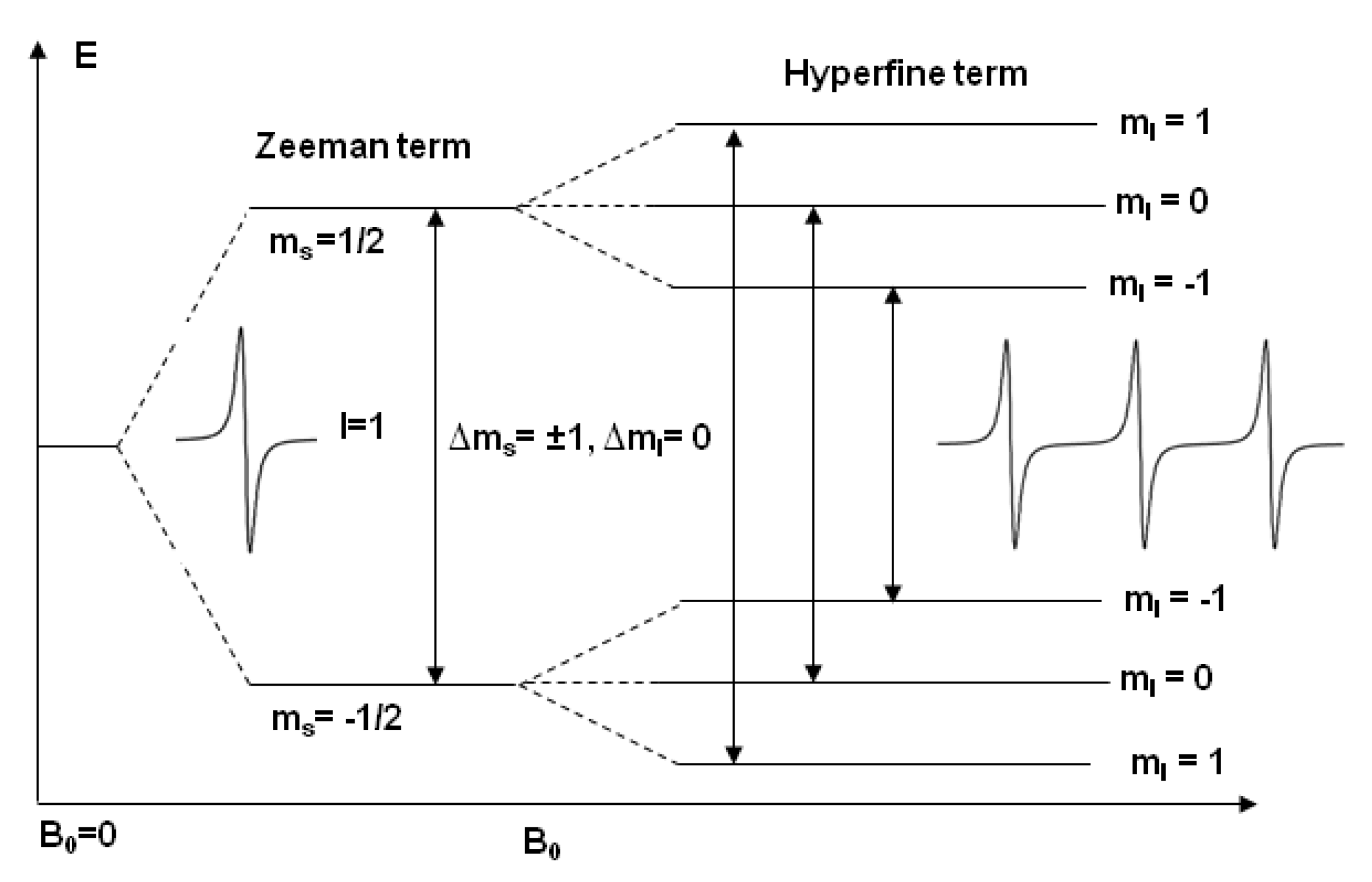
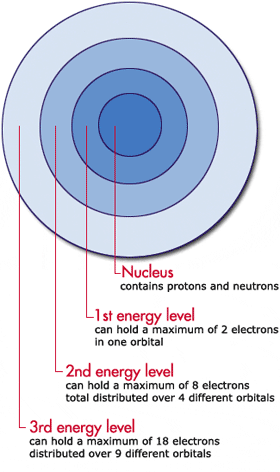
Chapter 1 INTRODUCTION TO NMR SPECTROSCOPY - University … 4 Introduction to NMR Spectroscopy Table 1.2. Properties of NMR Active Nuclei. Nuclei1 γ(rad·sec−1 · gauss−1)† INaturalAbundance(%) 1H26,753 1/2 99.980 2H4,106 1 0.016 19F25,179 1/2 100.0002 13C6,728 1/2 1.1083 31P10,841 1/2 100.00 1The term “Protons” is used interchangeably with 1Hinthetext. 2Fluorine is not normally found in biopolymers, therefore it … en.wikipedia.org › wiki › Electron_configurationElectron configuration - Wikipedia Lithium has two electrons in the 1s-subshell and one in the (higher-energy) 2s-subshell, so its configuration is written 1s 2 2s 1 (pronounced "one-s-two, two-s-one"). Phosphorus (atomic number 15) is as follows: 1s 2 2s 2 2p 6 3s 2 3p 3. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used. Electron configuration - Wikipedia Electron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.. An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that … structbio.pitt.edu › images › sbl2014Chapter 1 INTRODUCTION TO NMR SPECTROSCOPY or shielded, by the presence of electrons that surround the nucleus, giving a modified field at the nucleus, B: B =(1−σ)B o (1.4) where σ represents the degree of shielding. An extensive discussionofshielding effects is found in Section 1.3. Assuming that the magnetic field is along the z-axis, the energy of each state is: H = −u zB z ...
› cbse-sample-papers-for-class-10CBSE Sample Papers for Class 10 Science Term 2 Set 2 with ... Feb 10, 2022 · But it would require a large amount of energy to remove four electrons leaving behind a carbon cation with six protons in its nucleus holding on to just two electrons. 2. If carbon accepts 4 electrons, it becomes C 4-. But it would be difficult for the nucleus with six protons to hold on to ten electrons, that is, four extra electrons.
phet.colorado.edu › en › simulationBuild an Atom - Atoms | Atomic Structure | Isotope ... - PhET Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!


















:max_bytes(150000):strip_icc()/energylevels-56a129545f9b58b7d0bc9f39-5aeb7f1aae9ab800373981a3.png)











Post a Comment for "45 what is the term used to label the energy levels of electrons"