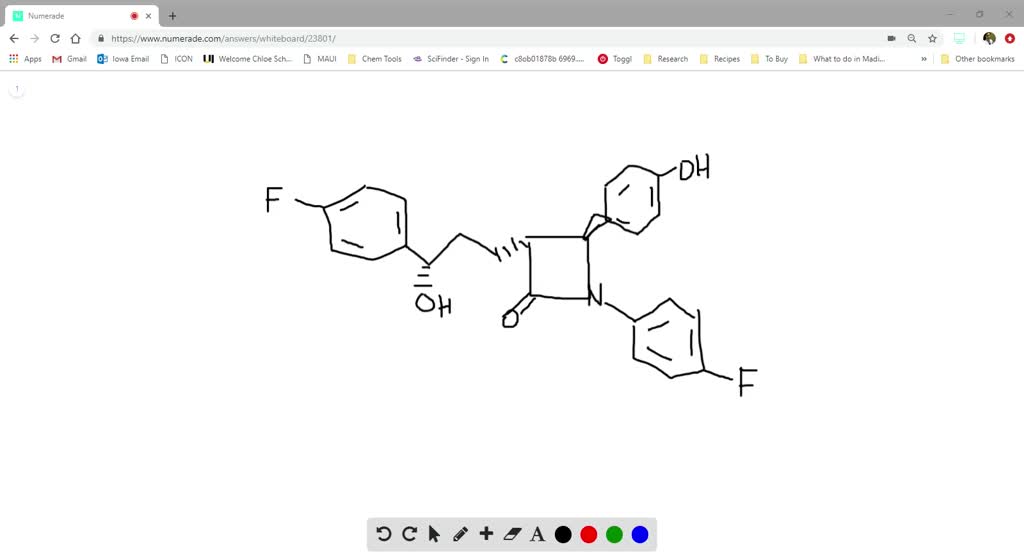
41 label each stereogenic center as r or s.
How to Assign R / S Configurations to Chiral Centers - dummies If a molecule has a chiral center that is designated R, the chiral center will be S in the molecule's enantiomer. You need to be able to assign whether a chiral center is R or S. To do so, you need to follow three steps: Number each of the substituents on the chiral center carbon using the Cahn-Ingold-Prelog system. Chemistry Department May 9, 2009 Organic Chemistry 233 ... I. Circle the correct answer in each of the following: (10 pts) ¾ The number of stereogenic centers in the molecule below is: N H CH 3 NH 2 OH a. 1 b. 2 c. 3 d. 4 e. 5 ¾ The number of possible stereoisomers of: CH CH Cl HO2C CH2 CH2 CO2H Cl
Answered: Problem 5.25 If the two stereogenic… | bartleby Label each stereogenic center as R or S (parts d, e and f please) Q8. Draw the two possible chair-like conformations of compound 1. Using the Cahn Ingold Prelog… R)-Lyrica has an optical rotation of -128 degrees in acetone solution and it's crystals have a… The question is based on the concept of Enantiomers.
Label each stereogenic center as r or s.
Stereochemistry configuration of r and s - slideshare.net It labels each chiral center R or S according to a system by which its substituents are each assigned a priority, according to the Cahn-Ingold-Prelog priority rules (CIP), based on atomic number. ... stereogenic center, the higher the atomic mass, the higher the rank. 2. If two or more atoms directly attached to the stereogenic center have ... Topic 5. Stereochemistry.pdf - Topic 5: Stereochemistry ... - Course Hero • This is done by adding the prefix R or S to the IUPAC name of the enantiomer. • To designate enantiomers as R or S, priorities must be assigned to each group bonded to the stereogenic center, in order of decreasing atomic number. • The atom of highest atomic number gets the highest priority (1).Labeling Stereogenic Centers with R or S Answered: Q2. Label all stereogenic centres as R… | bartleby Solution for Q2. Label all stereogenic centres as R or S. No explanation is required. H. CH3 CH2CH3 D INH2 N(CH3)2 H3CH2CO-
Label each stereogenic center as r or s.. CHEM 203 Stereochemistry Worksheet - CHEM 203:... Assign R / S configuration on each stereogenic center (chiral carbon). Draw a diastereomer of the molecule (Ph = benzene ring) Ph CH 3 Br Ph 2. Write the relationship between each of the following pairs of structures. ... Label each of the following molecules as 1. chiral or achiral ... Journal of the American Chemical Society | Vol 144, No 2 Highly dispersed dual Au and Cu species enable efficient and selective photocatalytic conversion of methane to methanol and oxygenates on ZnO using O2 as the oxidant operated at ambient temperature, resulting in a nearly 100% selectivity and 14.1% apparent quantum yield at 365 nm. View the article. SOLVED:Label each stereogenic center as R or S. - Numerade question as us to label each steer. Oh, center Azarias are so wonderful that we need to notice the lowest of the highest number in this case. Most of most of the cases hydrogen. If it's this line, then we keep the order. And if this light, then we reverse the artist. 5.6: Labeling Stereogenic Centers with R or S Stereocenters are labeled R or S The "right hand" and "left hand" nomenclature is used to name the enantiomers of a chiral compound. The stereocenters are labeled as R or S. Consider the first picture: a curved arrow is drawn from the highest priority ( 1) substituent to the lowest priority (4) substituent.
Chemistry 6.3 Flashcards | Quizlet For each constitutional isomer that contains a stereogenic center, draw all possible stereoisomers, and label each stereogenic center as R or S. Verified answer. CHEMISTRY. A redox reaction is a reaction in which: (1) only reduction occurs, (2) only oxidation occurs, (3) reduction and oxidation occur at the same time, (4) reduction occurs first ... Chapter 5 Assignment Saved 9 3 attempts l... - Physical Chemistry Chapter 5 Assignment Saved 9 3 attempts left Check my work Hint Be sure to answer all parts. 2 points Label each stereogenic center as R or S. CH3 CH(CH3)2 center 1 center 2 eBook Hic "CH3 HO SH References Stereocenter 1: (select) (select) Stereocenter 2: R S Worksheet#2_answers.pdf - Chem 204 Worksheet#2 ANSWERS 2 ... - Course Hero Label each stereogenic center as R or S For each molecule: [1] Convert the Fischer projection formula to a representation with wedges and dashed wedges. [2] Assign priorities. [3] Determine R or S in the usual manner. Reverse the answer if priority group [4] is oriented forward (on a wedge). 4. Assign R,S designations to each stereogenic center ... R/S - Two Stereogenic Centers R/S Naming Diastereoisomerism Meso Compounds Today, we'll look at naming compounds with stereocenters, and then we'll examine the complications which arise when a molecule has more than one stereocenter in it. First, though, let's look at a property in which one enantiomer differs from another. Enantiomers are alike in all respects but one.
7) (20 points) Label each stereogenic center as R or - Numerade HzN NHz HO- OH "OH Answer Label each stereogenic center as R or S. Discussion You must be signed in to discuss. Video Transcript question as us to label each steer. Oh, center Azarias are so wonderful that we need to notice the lowest of the highest number in this case. Most of most of the cases hydrogen. If it's this line, then we keep the order. PDF Priority Rules for Naming Chiral Centers - The R,S System atomic number of the atom that is bonded directly to the chiral center. The higher the atomic number, the higher the priority.! Number the four atoms, or groups of atoms, such that "1" has the highest priority and "4" has the lowest priority. 2. If two or more of the atoms that are bonded directly to the chiral center are the same, then Lone pair - Wikipedia The repulsive force of the oxygen atom's lone pairs pushes the hydrogens further apart, until the forces of all electrons on the hydrogen atom are in equilibrium. This is an illustration of the VSEPR theory. Dipole moments. Lone pairs can contribute to a molecule's dipole moment. NH 3 has a dipole moment of 1.42 D. Achiever Essays - Your favorite homework help service Eliza S. Australia, Victoria. Why Work with Us. Top Quality and Well-Researched Papers. We always make sure that writers follow all your instructions precisely. You ...
OCHEM Stereochemistry chpt 5 Flashcards | Quizlet An unknown compound X has two stereogenic centers that each have the R configuration. The diastereomer(s) of X will have the R AND S & S AND R configurations at the stereogenic centers. Stereoisomers are isomers that differe in their 3-dimensional arrangement of atoms.
Rearrangement - Michigan State University Cyclic ketones have two alpha-carbon atoms, each of which might shift to the nascent 1º-carbocation. If R = H in the case shown here, these two groups are identical and on shifting give the same product. If R = CH 3, the 2º-alkyl group shifts preferentially, the chief product being 3-methylcyclohexanone; the 2-methyl isomer is a minor product ...
SOLVED:Label the stereogenic center(s) in each drug as R or S. L-Dopa ... SOLVED:Label the stereogenic center (s) in each drug as R or S. L-Dopa is used to treat Parkinson's disease (Chapter 1). Ketamine is an anesthetic. Enalapril belongs to a class of drugs called ACE inhibitors, which are used to lower blood pressure. Oh no! Our educators are currently working hard solving this question.
Label Each Stereogenic Center As R Or S. - Modern Label Ideas The stereochemical labels r and s must be identical for each stereogenic center. If the substituents have multiple bonds the multiple bonded atoms are. If a molecule has a chiral center that is designated r the chiral center will be s in the molecules enantiomer. You need to be able to assign whether a chiral center is r or s.
Solved: Label each stereogenic center as R or S. | Chegg.com Organic Chemistry (4th Edition) Edit edition Solutions for Chapter 28 Problem 3P: Label each stereogenic center as R or S. … Solutions for problems in chapter 28 1P
Assign $R,S$ designations to each stereogenic center in glucose. This is two. This is three and Hodgins on this side, so we have to reverse the order. So 123 is cattle. Clockwise prefers is, huh? This is one again. Appears to marry is three. So 123 It is a car khweis. Reverse it. So this is s This is one to appear. Three now. Here. 123 Again, This is, uh, counterclockwise. So, uh, and one.

Post a Comment for "41 label each stereogenic center as r or s."